Welcome to the second of six articles in our analysis and compliance in pharmaceuticals series. In this post we explore how leading pharmaceutical companies are using assay by potentiometric titration to control variation and reduce risk during the drug manufacturing processes.
 Analysis of composition and quality is essential at every stage of the pharmaceutical manufacturing process, from formulation development to production. This is underscored by the FDA’s Quality by Design (QbD) initiative, in which product and process understanding is applied to preemptively control variation and reduce risk. It requires the thorough characterization of pharmaceutical products and the processes by which they are developed and produced.
Analysis of composition and quality is essential at every stage of the pharmaceutical manufacturing process, from formulation development to production. This is underscored by the FDA’s Quality by Design (QbD) initiative, in which product and process understanding is applied to preemptively control variation and reduce risk. It requires the thorough characterization of pharmaceutical products and the processes by which they are developed and produced.
Results of in-process tests are used to inform critical decisions made by formulators, and thus require accurate and timely results to facilitate efficient formulation development and production. They also ensure final material quality, from content to uniformity. Among the many analytical tests used to characterize a novel formulation, titration is one of the most economical, fastest, and most reliable techniques. Both active pharmaceutical ingredients (APIs) and excipients such as surfactants, edible oils, minerals, and chelating agents are addressed by titration and described in the U.S. Pharmacopeia (USP) monograph for roughly 630 APIs and 110 excipients. Titration also continues to be a relevant technique for assay and content uniformity of tablets.
Potentiometric titration in particular is a powerful tool in many facets of pharmaceutical manufacturing, from formulation development to process feedback to quality control of the finished product. The breadth of options in automation at every step of the titration process allows it to adapt to the various dosage forms, analytes, and goals needed to ensure tight control of incoming materials and high quality of finished pharmaceutical products. Automated titration offers many benefits over manual titration, providing more consistency and objectivity at each step, from sample preparation to determination of endpoint. It also improves the accuracy and repeatability of results, reduces waste of time and materials through human error, and increases the throughput of the analytical lab.
Assay of Active Pharmaceutical Ingredients
The characterization of a novel formulation requires distinct and varied analytical tests, both during processing and on the final material. The results of in-process tests are used to inform critical decisions made by formulators. Receiving timely results is key to efficient formulations development and production, as every tablet, vial, tube, or bag of product in a batch must have the same active substance content as stated on the packaging.
Various analytical methods are available for the exact determination of APIs, defined by the standard operating procedures in the European Pharmacopoeia and the USP. Current USP-NF monographs recommend potentiometric titration for assay of about 630 active pharmaceutical ingredients in both aqueous and non-aqueous media.
Case Study: Sulfanilamide
The purity of sulfanilamide, often employed for treatment of vaginal yeast infections, can be determined in aqueous solution by automatic, potentiometric titration using sodium nitrite as the titrant (Figure 1). Potassium bromide is added to the solution, as bromide ions act as catalyst for the diazotization titration. Using the Pt Titrode electrode, purity of the sample is determined in as little as three to five minutes, including time for electrode maintenance. Samples of fats and oils or components of acid-base mixtures often cannot be titrated in aqueous media. In these cases, solvent selection is key to obtaining accurate, repeatable results, particularly when isolating APIs from interfering excipients and carriers.
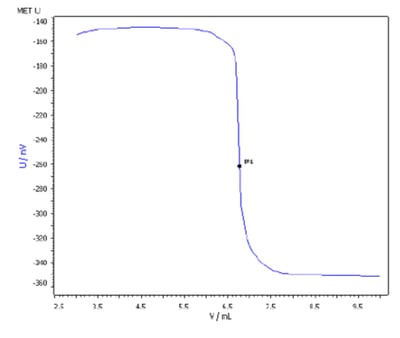
Figure 1: Titration of sulfanilamide using the Titrando 905, using sodium nitrite (Na NO2) = 0.1 mol/L as the titrant, hydrochloric acid, w(HCl) = 20%, and potassium bromide solution c(KBr) = 2.5 mol/L, with Pt Titrode, and temperature sensor. [Graphics note: Image taken from 2283144_ AN-t157]
Case Study: Ketoconazole
Ketoconazole is an antifungal drug used in the treatment of fungal infections and is prescribed as a tablet, cream (for ringworm or cutaneous candidiasis), or shampoo (for dandruff). Because of its low solubility point, less than 1 mg/mL, the concentration of ketoconazole can be determined by non-aqueous acid-base titration in three to five minutes, or up to 10 minutes including electrode conditioning time (Figure 2).
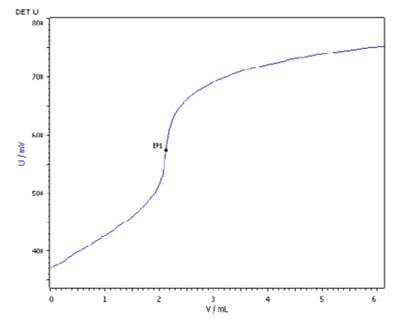
Figure 2: Titration of ketoconazole using the Titrando 907, using perchloric acid, c(HclO4) = 0.1 mol/L as the titrant, and Solvotrode easyClean as the electrode. [Graphics note: Image taken from 2073151_AN-t151]
Assay of Excipients
Excipients often comprise a large fraction of pharmaceutical products because they are used as fillers or bulking agents for formulations containing low concentrations of potent active ingredients. They also perform important functions like long-term stabilization and enhancement of the active ingredient. In the manufacturing process excipients can facilitate powder flow or non-stick properties, enhance stability of intermediates, and extend shelf life.
While the most common dosage forms of pharmaceuticals are tablets and injectables, many formulations are also delivered as aerosols, capsules, creams, films, foams, and gels. Excipients play a key role in formulation and need to be of high purity to meet the needs and regulatory requirements of the pharmaceutical industry. Although excipients are listed as inactive ingredients by the FDA, this class of compounds is often now used to impact the delivery and/or efficacy of the APIs, from reducing viscosity or enhancing solubility to facilitating drug absorption.
Excipients as a class are diverse, and often have uses other than in pharmaceutical applications. A supplier may discover that their product is being used by the pharmaceutical industry as an excipient, even if it was not originally intended for that purpose. In such cases, purity analysis of key excipients is imperative for successful formulation and batch to batch reproducibility, driving the need for accurate and repeatable test methods like potentiometric titration. Testing of excipients by potentiometric titration includes both raw material characterization assay and impurity testing. Current USP- NF monographs recommend potentiometric titration for assay for about 110 excipients, including surfactants, edible oils and lubricants, minerals, and chelating agents.
Surfactants
Surfactants, or surface-active agents, are widely used in pharmaceutical formulation as dispersing and solubilizing agents to increase the solubility and bioavailability of APIs. Surfactants are also used as emulsifying and wetting agents to maintain the pH or osmolality of liquid formulations. Additionally, surfactants are used in the pharmaceutical industry as antioxidants, emulsifying agents, aerosol propellants, tablet binders, and disintegrants; preventing aggregation or dissociation; and modulating immunogenic responses of active ingredients.
The concentration of anionic, cationic, and nonionic surfactants is a critical parameter and will often vary across formulations. The development of surfactants with improved capabilities leads to more complex matrices that are often challenging to evaluate. Specific surfactant electrodes are available for assay of each type of surfactant. As a result, potentiometric titration has grown to replace the classic, manual Epton titration method. A sample with a simple matrix or a raw substance can be analyzed in aqueous solution. Anionic surfactants are most often titrated using sodium dodecyl sulfate (sodium lauryl sulfate) as the titrant, buffer solution of pH = 3.0, or methanol as the reagent. Cationic surfactants are titrated using sodium dodecyl sulfate or formaldehyde solution as the reagent.
Nonionic surfactants are typically titrated using sodium tetraphenylborate (STPB), polyvinyl alcohol protective colloid, papaverine hydrochloride, sodium hydroxide solution, boric acid, or hydrochloric acid as the reagent. Titrants based on STPB are frequently used for the determination of nonionic surfactants and pharmaceutical compounds containing additives that significantly reduce deposition of precipitates formed during the titration on the electrode to reduce the frequency of electrode cleaning. Owing to the high surface affinity of cationic titrants, these must be added to the buret one day before use to ensure wetting of all glass parts and tubing that come into contact with the standard solution. Samples with a more complex matrix or which are not easily soluble in aqueous solvents are best analyzed with two-phase titration. Electrodes are available which are resistant to organic solvents and designed to be maintenance free. These include models optimized for use with chlorinated solvents and for samples with a high salt content and relative low surfactant content or measurements at pH values greater than 10 (e.g. soaps).
Case Study: Lidocaine in ointments
Lidocaine is an API used as an anesthetic and to counter arrhythmias. It can be assayed via potentiometric titration with sodium tetraphenylborate using a nonionic surfactant electrode. Methanol and heat are used to dissolve or destroy emulsion formulations, then glacial acetic acid is added to the prepared sample solution prior to titration with sodium tetraphenylborate. Here, automated potentiometric titration improves accuracy and repeatability of results while reducing human error.
Fats and Edible Oils
Fats, edible oils, and fixed oils are widely used in pharmaceutical formulation. Their rancidity will directly affect the shelf life and stability of the formulated products. USP chapter <401> recommends the following characterization tests for fats, fixed oils, waxes, resins, balsams, and similar substances, all of which can be determined by potentiometric titration (Table 1).
| Table 1 |
| Acid value |
| An acid value measurement corresponds to the quantity of carboxylic acid groups in fatty acids and is given in mg KOH per gram sample. The older an oil is, the higher the acid value, as triglycerides are converted into fatty acids and glycerol upon aging. If the results of a free fatty acids test are simply reported as acidity, without further definition this is, by convention, expressed as oleic acid. If the sample contains mineral acids these are, by convention, determined as fatty acids. |
| Ester value |
| The ester value is given in mg KOH required to react with the esters in one gram of a fat or oil. The ester value is the difference between the saponification value and acid value. |
| Hydroxyl value |
| Hydroxyl value is usually given in mg KOH required to neutralize the acetic acid taken up on acetylation of one gram of a sample containing free hydroxyl groups. |
| Iodine value |
| The determination of the iodine value is based on the addition of iodine to the double bonds of unsaturated fatty acids. The result is given as grams of iodine consumed by 100 g sample and is a measure for the unsaturation of an oil. Modern laboratories involved in routine process and quality control of fats and oils demand high analytical productivity with minimum operator involvement. Iodine value determination can be automated using the DIS- Cover technique and magnesium acetate as catalyst. The addition of the catalyst reduces the reaction time from 1–2 hours to 5 minutes. Peroxide value |
| Peroxide value |
| The peroxide number gives information about the number of peroxide compounds in the oil and the age and quality of the edible oil. The lower the peroxide number the better and/or newer the oil. Peroxide value is determined by redox titration using sodium thiosulfate titrant. Determination can be automated using the DISCover technique. |
| Saponification value |
| The saponification value is expressed as the quantity of KOH in mg required to saponify one gram of fat under the conditions specified. It contains the information of the average molecular weight of all fatty acids present. |
Pharmaceutical Water
Water is used as a solvent, vehicle, diluent, or filler for many pharmaceutical products. USP General Chapter <1231> on water for pharmaceutical purposes explains in detail the chemical testing needed for purified water, water for injection, water for hemodialysis, and pure steam. Chemical tests for these waters include accurate and reliable determination of pH, conductivity, alkalinity, hardness, and chloride. Prior to titration, the same electrode used for titration is often used to measure pH, and conductivity is often measured via a module connected to the titration system. An automated system for full water analysis including automated sample addition, automated calibration, and automated titer and cell constant determination—carried out in conjunction with automated titration systems—enhances reproducibility and minimizes manual error.
Assay by Potentiometric Titration with Colorimetric Endpoint Detection
Potentiometric titration with colorimetric endpoint detection is commonly used in the pharmaceutical industry as an alternative to manual visual color change titration. It offers fast, simple measurements based on a color change at the equivalence point and can be used with both aqueous and non-aqueous solvents. Some surfactants and several metals and metal oxides such as iron, nickel, cobalt, zinc, zirconium, aluminum, calcium, and magnesium are used in pharmaceutical formulations and titrated according to the USP using a photometric sensor as shown in the following case studies.
Case Study: Chondroitin sulfate
Chondroitin sulfate is a component of connective tissues found in cartilage and bone and is often used to treat osteoarthritis. It can be determined photometrically using hexadecylpyridinium chloride as the titrant with the Optrode at a wavelength of 660 nm (Figure 3).
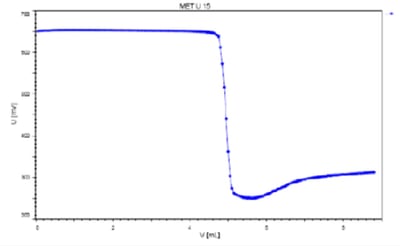
Figure 3: Titration of chondroitin sulfate sodium using the Titrando 907, using 1-hexadecylpyridinium chloride as the titrant, with Optrode (660 nm) as the sensor. (Graphics note: Image taken from 928186_ANt083)
Minerals and Chelating Agents
Minerals such as zinc, chromium, copper, iron, aluminum, zirconium, calcium, and magnesium are widely used in pharmaceutical formulations, over the counter products, and in dietary supplements. The purity of these minerals plays an important role, as they are used in pharmaceuticals (oral, parenteral, and topical formulations) to sequester ions from solution and to form stable complexes. Both the minerals and chelating agents’ purity can be easily determined using selective potentiometric titration with a colorimetric endpoint.
Case Study: Zirconium in pharmaceutical formulation
Zirconium is used in over the counter formulations with or without aluminum. Photometric titration offers a simple and straightforward method for its analysis. Zirconium is titrated directly with EDTA in acidic aqueous solution (buffer, pH 1). Eriochrome cyanine R is used as the indicator for this procedure. The equivalence point is determined with the Optrode at a wavelength of 520 nm.
Case Study: Automated determination of aluminum and magnesium in mixtures
Aluminum is commonly used in various antacid formulations. Aluminum can be selectively determined using photometric titration or potentiometric titration using copper ISE as an automated method. Mixtures of aluminum and magnesium ions can be analyzed by back titration at different pH values. The ion-selective copper electrode is used as the indicator electrode. First the aluminum is determined in acidic solution, then the magnesium in alkaline solution.
Assay and Content Uniformity of Tablets
Tablets are one of the most common dosing forms for pharmaceuticals, and yet can be one of the most challenging to analyze. Each tablet in a batch is required to have the same active substance content as that given on the packaging and within narrow limits. To achieve this, analysis is required at several stages. Mixing studies are performed during solid dosage form development and Figure 3: Titration of chondroitin sulfate sodium using the Titrando 907, using 1-hexadecylpyridinium chloride as the titrant, with Optrode (660 nm) as the sensor. (Graphics note: Image taken from 928186_ANt083) in manufacturing; a series of samples representing the uniformity of the mixers contents is collected and analyzed to provide feedback on the process and formulation. Postmanufacturing, tablet content uniformity studies validate on a statistical basis that every tablet of each batch contains the same solid dosage. While assays of APIs are often performed by UV-Vis or HPLC, modern potentiometric titration systems are a cost-effective option for automated analyses and provide relatively quick feedback to the manufacturing process.
 |
To achieve highly accurate results using potentiometric titration, sample preparation must be carried out carefully and reproducibly. Depending on the shape and coating of the tablets, the fillers they contain, and their API concentration, various sample preparation steps may be necessary before the analysis can be carried out. The first sample preparation step always includes the homogenization of the tablets in a suitable solvent mixture (Figure 4). Depending on the analysis technique, further steps such as diluting or pipetting may be required. Most laboratories carry out these tedious steps manually, allowing for carryover and contamination of the sample, leading to inaccurate results.
These problems can be eliminated by using a completely automatic system to standardize the sample preparation procedure. The same automated system can be used for the determination of the API concentration of a single tablet and to validate that the quality requirements have been met for the whole batch through random sampling. The automation available with modern potentiometric titration systems ensures high accuracy and reproducibility of results, increases sample throughput, improves safety in the laboratory, and reduces reagent consumption.
Complete automated processing of a tablet with subsequent titrimetric determination of the content of an ingredient can be performed in as little as eight minutes. Note that the time required for homogenization depends on the hardness and solubility of the tablets to be analyzed and is decisive for the total duration of the analysis. The only manual steps required are weighing out the sample, filling in the sample table, and placing the sample on the sample changer rack. The remaining steps occur automatically. Automation prevents any changes to the water content of the sample during preparation, and in some cases, it can also eliminate the need for toxic solubility promoters.
Frequency of Sampling
The USP monograph on content uniformity stipulates testing of solid dosage form samples at a frequency of 10 samples per batch. While this provides a solid baseline to validate dosage, interest has increased in cost-effective, time-efficient methods for tablet assay and content uniformity testing as indicated by the FDA’s process analytical technology (PAT) initiative.
While HPLC is very accurate, analyzing 10 tablets for content uniformity could take hours – and the results may not be available to the tablet press operators or for batch release for many days or even weeks after the tablets are compressed. Potentiometric titration offers much faster feedback for the assay of many APIs, facilitating the analysis of content uniformity in tablets at a rate that is beneficial to production.
Case Study: Benzbromaron
Benzbromaron is the API used in a common tablet administered to lower an increased uric acid level in the blood in the treatment of gout. In addition to sophisticated and expensive LC-MS and GC-MS methods, benzbromaron can be effectively determined by titration with sodium hydroxide solution using a straightforward, fully automated sample preparation. Benzbromaron is a weak acid whose pKa (4.50) is comparable to that of acetic acid (4.75). After the loss of the hydrogen ion, the negative charge at the oxygen atom is delocalized around the ring (resonance stabilization). The more stable the ion is, the more likely it is to form. Hence, titration with strong bases is a convenient method for benzbromaron determination. The total determination, including sample preparation, takes only eight minutes. A 10-fold determination of samples containing one tablet produced an active substance content of 99.2 mg per tablet, in excellent agreement with the 100 g per tablet value given by the manufacturer (Figure 5).
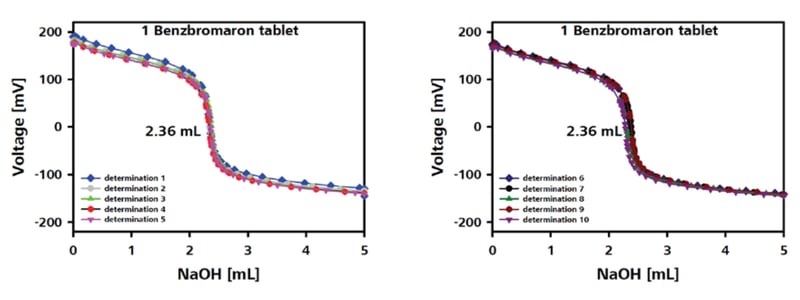
Figure 5: Titration of chondroitin sulfate sodium using the Titrando 907, using 1-hexadecylpyridinium chloride as the titrant, with Optrode (660 nm) as the sensor. (Graphics note: Image taken from 928186_ANt083)
Conclusion
Potentiometric titration is the most common approach for automated titrations. In combination with automated sample preparation, it has both increased efficiency and reduced manual work load in many pharmaceutical labs. Each sample is handled in exactly the same way, increasing the accuracy of determinations, simplifying overall workflow, and delivering consistently accurate results. It has become a powerful technique for the analysis of active ingredients and excipients in pharmaceutical and personal care products. These factors have earned potentiometric titration a place as a go-to technique for analysis of the composition and quality of pharmaceuticals. By providing both rapid in-process feedback and accurate quality control, it will remain a mainstay of the industry as it advances in its Quality by Design efforts.
References
- AB-268: Potentiometric titration of surfactants and pharmaceuticals – an overview.
- AN-T-109: Automated determination of the iodine value.
- 8.000.6061: Applications of automated thermometric titrimetry in routine process and quality control of fats and oils.
- AB-141: Analysis of edible fats and oils.
- AN-T-110: Automated determination of the peroxide value.
- AN-T-111: Determination of the saponification value.
- AN-T-148: Determination of zirconium using automatic photometric titration.
- AN-T-117: Automatic determination of aluminum and magnesium mixtures with ion-selective copper electrode (Cu ISE).
Posted by Kerri-Ann Blake, Ph.D.
Dr. Kerri-Ann Blake is a Product Specialist at Metrohm USA in Riverview, Florida. She earned her PhD in Materials Science and Engineering at the University of Florida where she was a researcher at the Particle Engineering Research Center. Kerri-Ann joined the Applications team at Metrohm USA in 2012 focusing on titrations. She has since expanded her portfolio of technologies to include Electrochemistry, Spectroscopy, and Process Analytics. She performs internal as well as customer training. Kerri-Ann also has experience with writing Karl Fischer ASTM methods and has participated in their corresponding interlaboratory studies.