Welcome to the third of six articles in our analysis and compliance in pharmaceuticals series. This article explores how automated volumetric and coulometric Karl Fischer titration and the KF oven method are used to determine water content in products throughout drug manufacturing.
 Many active pharmaceutical ingredients (APIs) and adjuvants contain water in an adsorbed form (surface water) or bound as a hydrate (water of crystallization). The water content of pharmaceuticals strongly influences their quality, shelf-life, and stability as well as the release of the active substances. The determination of water content is, therefore, vital in pharmaceutical analysis.
Many active pharmaceutical ingredients (APIs) and adjuvants contain water in an adsorbed form (surface water) or bound as a hydrate (water of crystallization). The water content of pharmaceuticals strongly influences their quality, shelf-life, and stability as well as the release of the active substances. The determination of water content is, therefore, vital in pharmaceutical analysis.
The European Pharmacopoeia, 4th Edition (2002), describes various methods for determining the water content of pharmaceuticals and highlights the Karl Fischer titration method. In cases of high water content, the method is carried out volumetrically (semi-micro determination). For substances with a very low water content a coulometric Karl Fischer titration (micro-determination) is performed. Automatic Karl Fischer water determination offers advantages over alternative, manual, time-intensive methods. Here you will discover how the KF oven method in combination with the KF coulometer offers automated features that increase accuracy, repeatability, and efficiency when determining water content of various low moisture substances. In addition, you will learn how volumetric KF titration in combination with a high-frequency homogenizer determines water content of tablets quickly and accurately.
Karl Fischer Oven Method
 Substances that release water slowly, at high temperatures, or exhibit low solubility in alcohols are not suitable for a direct Karl Fischer titration. In these cases the KF oven method can be employed to overcome challenges associated with traditional methods involving the use of toxic solvents, extensive sample preparation procedures, or the release of impurities when using a drying cabinet or desiccator. With the automated KF oven method, the substance under investigation is heated in a tube oven and the released water is transferred by a carrier gas to the titration cell where it is then determined by Karl Fischer titration. Only the water enters the KF cell, and the sample itself does not come into contact with the KF reagent eliminating unwanted side reactions and matrix effects.
Substances that release water slowly, at high temperatures, or exhibit low solubility in alcohols are not suitable for a direct Karl Fischer titration. In these cases the KF oven method can be employed to overcome challenges associated with traditional methods involving the use of toxic solvents, extensive sample preparation procedures, or the release of impurities when using a drying cabinet or desiccator. With the automated KF oven method, the substance under investigation is heated in a tube oven and the released water is transferred by a carrier gas to the titration cell where it is then determined by Karl Fischer titration. Only the water enters the KF cell, and the sample itself does not come into contact with the KF reagent eliminating unwanted side reactions and matrix effects.
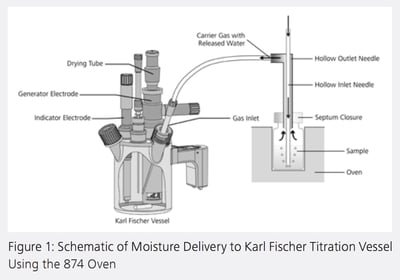
Using the 874 Oven Sample Processor, analytes are weighed directly into sample vials, sealed tightly, moved by the turntable to the appropriate position above the oven, and then lowered automatically into the heating block. carrier gas, loaded with the released moisture, then flows through the outlet needle to a heated transfer tube directly into the titration cell, where the Karl Fischer water determination takes place (Figure 1). Since the Carrier gas passes directly through the sample, instead of passing over it, a more complete, accurate amount of water is released.
The following case study highlights the benefits of the automated KF oven method using the 874 Oven Sample Processor and the 851 KF Coulometer. Table 1 lists the equipment and reagents used in the study.
Case Study: KF Oven Method
| Table 1. Instruments, Accessories, and Reagents |
| 874 Oven Sample Processor |
| 851 KF Coulometer, including KF cell without diaphragm |
| 803 Magnetic Stirrer. 6.5617.000 complementary equipment for automatic reagent exchange 800 Dosino |
| tiamo 2.5 software for data acquisition, storage, and reprocessing |
| Reagents: |
| Hydranal Coulomat AG Oven |
| Hydranal Water Standard KF Oven |
| Nitrogen as inert carrier gas |
Using the KF oven method, approximately 40 pharmaceuticals from the European Pharmacopoeia were analyzed. The investigated pharmaceuticals were substances with a defined water content, some of which undergo side reactions with KF reagents and therefore cannot be analyzed by direct Karl Fischer titration. Between 15 and 30 mg of the pharmaceutical is weighed directly into each sample vial, which is then hermetically sealed with PTFE-coated septa. Here, manual sample preparation is reduced to a minimum, and significantly less product is used as compared with traditional methods which require a sample size of one gram or more. Smaller sample size also allows for lower reagent consumption during titration.
At least a threefold determination is carried out on each substance allowing for highly reproducible analysis conditions and improved repeatability of results. Prior to each determination the complete system is conditioned until a constant low drift (approx. 10µg/min) is attained. During this procedure the needle is located in a special conditioning vessel on the rack of the Oven Sample Processor.
To obtain accurate results, the blank (three empty sample vials) is analyzed to account for moisture adhering to the vessel walls, vial cap, and septum. In addition, the complete system is checked at regular intervals with a certified KF standard.
Analysis Temperatures
To determine the appropriate analysis temperature, the Oven Sample Processor allows temperature gradients to be run. Using the recorded water-release curve, the optimum analysis temperature is determined. Here both thermal stability (instability) of the substance and the fact that water is only released at a sufficiently rapid rate at temperatures above 100C are taken into account. The oven temperature should be set as high as possible to ensure short determination times but remain 20 to 30C below the decomposition temperature.
The analysis temperatures are determined on the basis of the water release curves that were recorded for all the investigated pharmaceuticals in the temperature range 50 - 250C. In addition, all the pharmaceuticals were examined by means of a Kofler microscope, and their melting points were determined. This instrument allows the substance to be closely observed during the heating and melting phases; any alterations such as color changes, sublimation, or decomposition reactions can be easily recognized.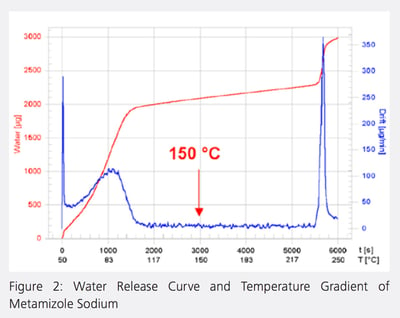
Figure 2 shows the water release curve and temperature gradient of metamizole sodium. Metamizole sodium melts at 220 to 221C with decomposition. Water determination by direct volumetric or coulometric KF titration is not possible because the substance is oxidized completely or partially by iodine. The water release curve was recorded using a heating rate of 2C/min: the sample was heated from 50 to 250C in 100min. Both the surface moisture and the water of crystallization are released within the time interval 0 - 1600s (50 - 103C). The drift then falls to its original value of approximately 10µg/min and remains virtually constant for 3800s. Starting at 5400s (230C) both curves show a steep increase. Evidently, water is released by decomposition from this temperature onward.
A temperature from the central region of the plateau of the red curve (150C) was selected as the oven temperature for determining the water content of metamizole sodium, ensuring that the water is released quickly and completely without decomposition.
Case Study Results
Table 2 shows the six most important substances among the 40 investigated pharmaceuticals (the water contents shown are the mean values of threefold determinations). For comparison the table also contains the theoretical (calculated) water contents of the substances and information about the loss on drying given in the European Pharmacopoeia. Analyses are performed in 10 to 12 minutes as compared to several hours in traditional drying cabinet methods. Further, the KF oven method precludes release of other volatile species, thus ensuring accurate results.
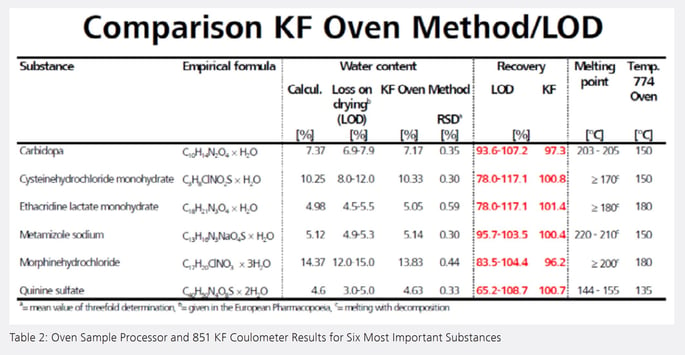
The water contents determined with the 874 Oven Sample Processor and 851 KF Coulometer all lie within the ranges specified in the European Pharmacopoeia. The Pharmacopoeia gives a wide recovery range for the loss on drying. In the case of quinine sulfate, an antimalarial drug, a range between 65.2 and 108.7% is specified based on the theoretical (calculated) water content. The oven system yields an excellent recovery of 100.7% for this substance. When all the investigated pharmaceuticals are considered, the recovery using the KF oven method lies between 90 and 110%. High repeatability was also obtained with relative standard deviations lying between 0.30 and 2.0%.
Automated Homogenization for Water Content in Tablets
The quality, hardness, compaction, and shelf-life of pharmaceuticals depend largely on their water content. Most pharmacopoeias stipulate thermogravimetry and Karl Fischer titration for water quantification. The former method requires labor-intensive sample preparation steps and leaves considerable margin for error, and tablets often have limited solubility in Karl Fischer working media. Using an automated, high-frequency homogenizer that also serves as a stirrer during titration significantly reduces manual sample preparation and allows for volumetric KF titration of released water.
The system for the automated determination of the water content by volumetric KF titration consists of the 901 Titrando, the 815 Robotic USB Sample Processor XL with two towers, and Polytron with comminution aggregate (high-frequency homogenizer). The Polytron homogenizer is mounted on the robotic titration head of the sample processor tower and is adjusted to the correct working height. The second tower is used for emptying the sample beakers after the determinations, reducing reagent handling to a minimum. All instruments are controlled by the tiamo™ software.
Once the system is set up, the dosing devices are preflushed to displace air bubbles and moisture. Four blank determinations of working solution without sample are carried out; the first one is the system preparation value, the latter three provide the mean blank value. The titer of a commercially available water standard is determined (n = 3). A defined number of tablets is directly weighed out into the sample vessel, the samples are then placed on the sample processor rack, and all relevant data (sample weight, sample identification) are entered into the tiamo™ software. All sample vessels are sealed with aluminum foil and a sleeve. The working medium is transferred to the sample vessel, and the Polytron comminutes the tablets. Comminution speed and time depend on tablet size and hardness and were determined in preliminary experiments. The released water is titrated with KF reagent at a stirring speed of 7500 rpm. After each determination a cleaning step with methanol is performed to avoid sample material carry-over; in order to prevent cross-contamination, the methanolic cleaning solution is titrated to dryness.
Linearity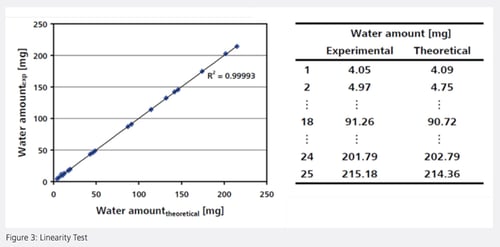
A linearity test in the range of 4…215 mg was performed with the sodium tartrate dihydrate standard. The experimentally determined amount of water agreed very well with the theoretical amount, resulting in an outstanding coefficient of determination (Figure 3).
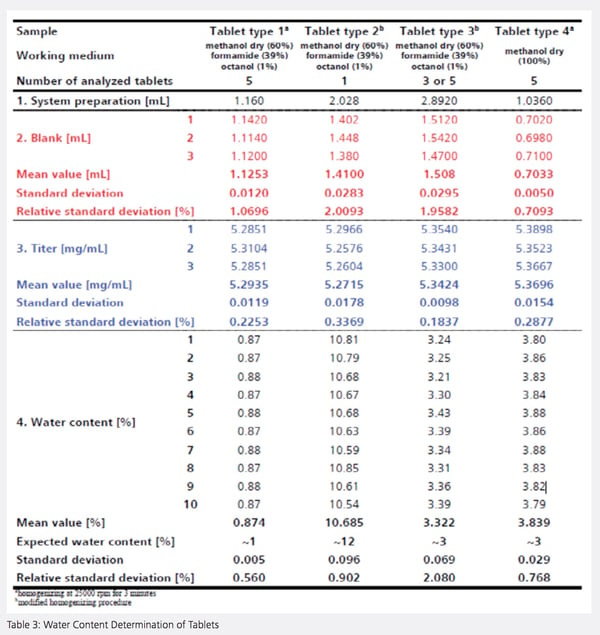
Four tablet type samples were analyzed to determine moisture content using the automated KF homogenizer system. In addition to determining the system preparation value, the blank, and the titer, ten determinations were carried out. The determined water contents were all within the range expected by the manufacturer and corresponded to previously validated values (See Table 3).
Conclusion
In cases where direct KF titration is not possible, employing the KF oven method and using automated homogenizers for tablets provide solutions for automatically determining water content of challenging samples. Using an automated oven sample processor in combination with an automated KF coulometer reduces sample size, increases sample throughput, and improves accuracy and repeatability for many low moisture content pharmaceuticals. Employing an automated, high-frequency homogenizer in combination with KF volumetric titration allows for accurate, highly repeatable water content determination of various tablet samples.
References
- AB-268: Potentiometric titration of surfactants and pharmaceuticals – an overview.
- AN-T-109: Automated determination of the iodine value.
- 8.000.6061: Applications of automated thermometric titrimetry in routine process and quality control of fats and oils.
- AB-141: Analysis of edible fats and oils.
- AN-T-110: Automated determination of the peroxide value.
- AN-T-111: Determination of the saponification value.
- AN-T-148: Determination of zirconium using automatic photometric titration.
- AN-T-117: Automatic determination of aluminum and magnesium mixtures with ion-selective copper electrode (Cu ISE).
Posted by Kerri-Ann Blake, Ph.D.
Dr. Kerri-Ann Blake is a Product Specialist at Metrohm USA in Riverview, Florida. She earned her PhD in Materials Science and Engineering at the University of Florida where she was a researcher at the Particle Engineering Research Center. Kerri-Ann joined the Applications team at Metrohm USA in 2012 focusing on titrations. She has since expanded her portfolio of technologies to include Electrochemistry, Spectroscopy, and Process Analytics. She performs internal as well as customer training. Kerri-Ann also has experience with writing Karl Fischer ASTM methods and has participated in their corresponding interlaboratory studies.