Welcome to the first of six articles in our analysis and compliance in pharmaceuticals series. In this post we explore how leading pharmaceutical companies are implementing handheld Raman spectroscopy into their drug manufacturing processes.
 Raman spectra provide a data rich signal from which materials can be identified or verified with high accuracy, and little or no sample preparation. Direct analysis of incoming and outgoing materials at the warehouse and on the manufacturing floor is cost effective and provides increased traceability, eliminating the need to send materials to an analytical laboratory. These capabilities enable handheld Raman spectroscopy applications throughout the manufacturing process and result in increased productivity and lower production costs.
Raman spectra provide a data rich signal from which materials can be identified or verified with high accuracy, and little or no sample preparation. Direct analysis of incoming and outgoing materials at the warehouse and on the manufacturing floor is cost effective and provides increased traceability, eliminating the need to send materials to an analytical laboratory. These capabilities enable handheld Raman spectroscopy applications throughout the manufacturing process and result in increased productivity and lower production costs.
Despite these advantages, smooth implementation of handheld Raman across the manufacturing line remains a challenge due to the differing expectations from operators, developers, and facility or corporate management. Fulfilling the expectations of these three important groups in the manufacturing environment is the key to successful implementation of handheld Raman. This article sheds light on recent advances in sampling and instrument management that better meet the needs of all users.
Handheld Raman Spectrometers
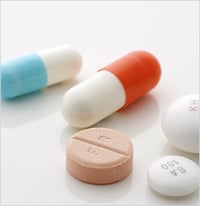
Lab-based spectroscopy techniques; whether FTIR, Raman, or other technology, require the same traditional workflow of sampling, labeling, and transporting the sample to the analytical lab. Traditional lab-based Raman instruments are expensive and primarily used for research and development by spectroscopists. Handheld and portable Raman systems bring this analytical capability into mainstream activities. Implementing handheld Raman can significantly streamline quality control measurements by eliminating this traditional workflow and measuring in situ. Since the introduction of the first widely successful handheld Raman spectrometer, different sectors of the pharmaceutical and chemical industries have moved to adopt handheld Raman as a screening tool to replace traditional lab-based analysis methods. Handheld Raman instruments are now used as identification, verification, and screening tools across manufacturing lines for raw materials, in process samples, and finished products. (Figure 1)
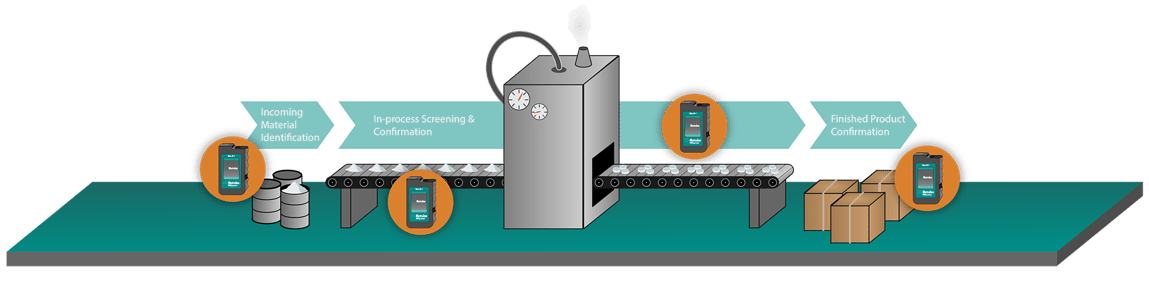
Figure 1: Examples of places where handheld Raman can be implemented in manufacturing.
Measurement traceability and cost-effectiveness are critical drivers of handheld Raman implementation across industries as problems with poor quality raw materials, product adulteration, and counterfeit ingredients proliferate. In the pharmaceutical industry, traceability and incoming material inspection is mandated by FDA regulations. Other sectors are following suit with the implementation of cGMP and cGLP standards as a strategy to ensure quality.
Successful Handheld Raman Implementation
A major factor for successful implementation of handheld Raman comes with providing training for operators and gaining an understanding of technical and financial benefits for method developers and managers. The features and benefits that appeal to the end user sometimes differ from features that appeal to spectroscopists doing method development and upper management. The end-user may receive hundreds of incoming containers per day in a facility that may have or process thousands of materials. These operators may have limited technical education, receive minimal training, and must maintain a high operational tempo. In this environment easy to use instruments help maintain adequate workflow. Method developers are most concerned with compliance features, instrument specifications, error rates, and overall result reliability. At the management level, other requirements such as overall return on investment, instrument reliability, and the ease of deploying and managing multiple instruments are primary concerns.
Technology for Operators
The underlying technology for handheld Raman has advanced, resulting in systems that do more than verify raw materials and identify unknowns. They now identify multicomponent mixtures and can make quantitative measurements. Even fluorescence, long the bane of Raman spectroscopy at 785nm, is being mitigated. The appearance of instruments with longer excitation wavelengths (above 1000 nm) or multiple wavelength excitation promise to extend handheld Raman’s reach into increasingly difficult materials and matrices. Recently developed 532 nm systems promise to make handheld Raman more applicable to biopharmaceuticals, which are often in aqueous solutions. With the recent advent of spatially offset Raman spectroscopy instruments, rapid through-package screening is now available. Materials that should not be opened can now be more easily identified and qualified.
Despite these technological advances, user-to-user variation and material/packaging variety are still challenges encountered when handheld Raman is used along the manufacturing line. These challenges are mitigated by a combination of successful sampling strategies and continual end-user education. To address the challenges associated with sampling, instrument manufacturers are constantly improving the feature sets of their hardware and software to improve the data reliability while maintaining the usability that has made this family of tools successful.
 |
Examples include the incorporation of mixture deconvolution algorithms, new sampling methods such as immersion probes, and even smart scan technology that automatically optimizes the signal. A major challenge for operators can be variability of the Raman signals due to sample damage or material heterogeneity. Many organic materials and biopharmaceuticals degrade when exposed to 100+ mW laser beams with sub millimeter diameters for 10 to 30 seconds.
Since high laser powers are required to keep integration times short, there are two strategies for mitigation. One is to emit a large diameter laser beam, which reduces the power density, but requires larger collection optics for efficiency. A larger beam simultaneously compensates for material heterogeneity by measuring a macroscopic area. The other strategy is to raster the Raman laser beam across a small area of the sample at high speed. (Illustrated in Figure 2) This raster technology prevents the laser from being incident on any area too long, avoiding damage. It accounts for heterogeneity by spatially averaging the sample. Because the spot size remains small, the size of the optical train can be reduced without sacrificing spectral resolution (1).
The first step to reliable Raman, anywhere in the manufacturing line, is selection of the correct sampling accessory (Table 1). Standoff objective lenses, or point and shoot accessories, are the most commonly used sampling tools for handheld Raman spectroscopy in pharma. Rather than holding the sample at some specified distance from the instrument, these tools make direct contact with a container to provide a more consistent sample exposure. To use them correctly, simply press the objective against the surface of a bag or bottle and wait until the analysis is complete. Because the lenses come in a variety of focal lengths, it is important to choose the right lens for each application. Short working distance (SWD) objectives typically have focal lengths around 1mm and are best used to scan through transparent and thin packaging, such as plastic bags and drum liners. Long working distance (LWD) accessories, sometimes called bottle adapters, have typical working distances of 5-10 mm and place the focal point, where the Raman signal is strongest, beyond the contact surface. LWD accessories measure inside transparent plastic and glass bottles, without having to open them.
| Table 1 | |
| Sampling Accessories and Applications | |
| Sampling Accessory | Application |
| SWD Objective | RMID through 1-3 layers of plastic bags |
| LWD Objective | RMID through bottles |
| Vial Holder | RMID of liquids when a sample must be pulled |
| Performance BallProbe® | RMID of hard to reach areas. Top/middle/bottom sampling. |
| Tablet Holder | Counterfeit detection |
To further improve measurement reliability and consistency with standoff objectives, the use of vial holders and tablet holders is recommended. Liquids or powders are transferred to disposable glass vials and inserted into a special holder that reproducibly positions the vial, avoiding operator error by stabilizing the measurement location. Tablet holders provide reproducible positioning of a tablet to the Raman sensor. Because of the wide variety of tablet shapes and sizes, reproducibly scanning tablets with a standoff objective can be challenging, although necessary if the material cannot be removed from a blister pack. Some tablet holders require manual positioning and tightening of screws which allow for a high degree of operator control but may pose challenges for high throughput applications. Other instrument manufacturers provide spring – loaded holders that allow for quicker operation and reproducible placement.
 |
In applications where direct measurement is preferable, immersion probes bring the sample into direct contact with the probe, thereby eliminating human error from mispositioning the standoff objective. Immersion probes also minimize sample handling and provide a way to rapidly evaluate large numbers of samples. Figure 3 shows an example of an immersion probe in use.
The Raman probes for handheld instruments currently available have long (20 –36 cm) stainless steel shafts with sapphire windows that can take direct measurements inside liquid containers such as barrels, drums, and bottles. Sapphire is both scratch and chemical resistant, making it well suited to the manufacturing or warehouse floor. Probes such as those developed by MarqMetrix and licensed by Metrohm for handheld Raman have an exposed sapphire sphere at the tip, which gives a very short focal length of around 0.250 mm. The small depth of field and the exposure of the sapphire sphere make this technology well suited for analyzing solid samples and small amounts of materials on surfaces with which the probe is in direct contact. This also enables top-middle-bottom sampling of containers through a thief.
Handheld Raman instruments with probes may also be directly inserted into the production lines through a sample thief or other appropriate provision to provide rapid spectral data for process monitoring and improvement. For example, blend homogeneity can easily be monitored with Raman spectroscopy, and reaction monitoring has been shown to be a viable application for handheld instruments (2). While process monitoring can also be accomplished with viewports and standoff objectives (3), immersion probes minimize the impact on the manufacturing line.
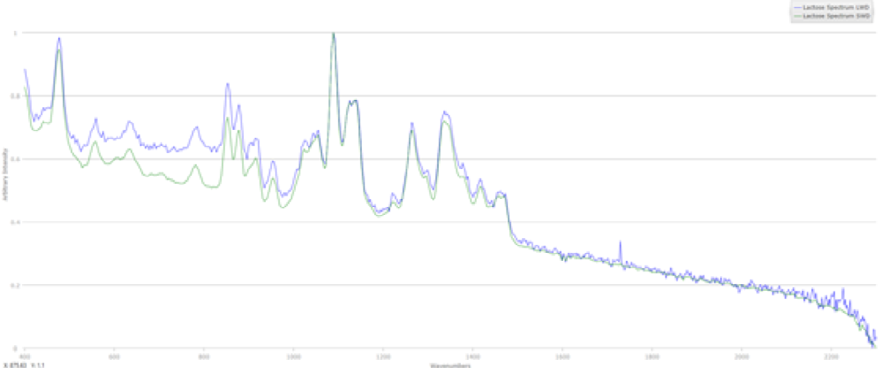
Figure 4: Spectra collected of lactose monohydrate with a (green) SWD and (blue) LWD lens with a Mira.
The LWD data has a much lower signal to noise ratio.
Technology for Method Developers
As a method developer, algorithms that work in the lab must translate into durable operating procedures along the manufacturing line. This means minimizing operator driven errors and ensuring consistent instrument function. For example, both Bruker and Metrohm have added coded memory chips to their sampling accessories so that their instruments know which accessory collects each measurement. These accessories can be locked to a particular method, ensuring the use of the correct sampling tool and avoiding errors (Figure 4). The sample accessory information is recorded in the instrument audit trail to provide additional measurement traceability.
In addition, data collection and analyses are enhanced by available automations to sample naming. Batch scanning allows the naming parameters to be configured and then left alone on the instrument until the task is complete. Many of the instruments available today also have integrated barcode readers. These can be configured to automatically populate sample name, lot, and batchfields with information according to how they are configured in software. Barcode scanning and task automation ensure that accurate sample information is captured and signed, reducing post-analysis correction time.
Many industries use materials that do not yet exist in qualified libraries provided by instrument manufacturers. With open library structures, developers can create qualified library elements that make it easier to identify and build verification methods that capture the variance of the acceptable raw materials. The robustness of these methods can be further enhanced by implementing additional controls on operating procedures. Examples of enhanced controls include configuration of measurement time, sample averaging, laser power, and even algorithm thresholds. These features can ensure that every analysis is performed exactly as it was in the laboratory, thus preventing sample degradation or enhancing the spectrum from a low signal material.
Implementing Handheld Raman Across Multiple Locations
Higher levels of instrument performance available through operating procedure controls in newer instruments make it easier to deploy instruments across multiple sites. Procedures can be transferred between a development instrument and others through a validated instrument method transfer protocol as outlined in USP <1224>. Operating procedure transfer can overcome the challenges of calibration survivability and variations in automated method transferability among manufacturers. Each measurement no longer has to be independently validated on each instrument to ensure consistent results across sites, thus saving time and the overall amount of validation required.
Conclusion
Software and hardware innovations in sampling and instrument control are increasing the reliability of handheld Raman measurements, making the technology suited for a wider number of users and applications. High quality, simple sampling accessories optimized for different applications deliver rapid and reproducible results. Microchips, barcode readers, and the seamless integration of hardware and software allow handheld Raman to be implemented by a variety of users for applications ranging from incoming materials inspection, to production monitoring, to finished product inspection.
References
- Watson, M.; Buller, S.; Carron, K. US Patent 8988678 B2. 2011.
- Padlo, T. and Bakeev, K. Spectroscopy 31(9) p16-22.
- Hopkins, A. J. “Implementation of Handheld Raman for Online and at Line Applications.” IFPAC 2017.
Posted by Adam J. Hopkins, Ph.D.
Adam has a Ph.D. in chemistry from the University of Oregon and nearly 15 years of experience in vibrational spectroscopy. Prior to joining Metrohm USA, Adam spent five and a half years developing spectroscopic solutions for standoff and noncontact sensing applications. Dr. Hopkins is a member of the American Chemical Society, Society for Applied Spectroscopy, Coblentz Society, and SPIE. He is the author of 11 papers and proceedings, and has three patents pending.