Welcome to the fifth of six articles in our analysis and compliance in pharmaceuticals series. This post explores near-infrared spectroscopy analysis in pharmaceutical manufacturing, its versatile applications including material identification and quality assurance, for cost effective and time saving results.
Brief History of Near Infrared Spectroscopy in Pharmaceuticals
 Near-infrared spectroscopy (NIRS) is recognized by common pharmacopoeias as a secondary method for a fast and reliable, non-destructive analysis in pharmaceutical manufacturing. Since the 1960’s it has been explored and used in the pharmaceutical industry for raw material identification, process control, and quality assurance of final products. Because quality control plays a central role in the pharmaceutical industry and is based on regulatory requirements and guidelines, the need for an approach like NIRS has become a valuable tool.
Near-infrared spectroscopy (NIRS) is recognized by common pharmacopoeias as a secondary method for a fast and reliable, non-destructive analysis in pharmaceutical manufacturing. Since the 1960’s it has been explored and used in the pharmaceutical industry for raw material identification, process control, and quality assurance of final products. Because quality control plays a central role in the pharmaceutical industry and is based on regulatory requirements and guidelines, the need for an approach like NIRS has become a valuable tool.
Its versatility, reliability, and ability to meet validation requirements make NIRS “a highly relevant tool for achieving control when builtin quality is preferred over quality by testing” by the U.S. Food and Drug Administration (FDA), the Process Analytical Technologies (PAT) initiative, guidelines by the European Medicines Agency (EMA), and the International Conference on Harmonization in the standards (ICH) Q8(R2), ICH Q9 and ICH Q10 (1). Here, you’ll discover how Metrohm solutions are applied and validated across the pharmaceutical industry to comply with regulatory requirements.
NIRS Applications
NIRS is a versatile analysis method and can be used for a vast number of applications throughout the pharmaceutical manufacturing process. Here example applications will be explored across the manufacturing line, from inspection of raw materials, to inline/online process control, to product development, to quality control of finished products.
Incoming Materials Inspection
According to FDA CFR 211.84 and EU GMP 8, all incoming materials are tested to verify identity and conformity. The Metrohm spectrometer product portfolio offers suitable solutions for a convenient inspection of large quantities and varieties of incoming materials—such as multiple varieties of medicinal plants used as raw materials— whether they are analyzed directly in the warehouse, in the weighing area, or in the QC lab (2, 3).
Inline/Online Process Control
Metrohm NIRS inline/online analyzers offer real-time monitoring and optimization of residual solvent and water content in powders and granulates, such as in lyophilized products. More specifically, inline monitoring of the blending, granulation, and drying of lactose anhydrous and starch 1500 with residual methanol, can occur using an NIRS XDS Process Analyzer fitted with a fiber optic probe. The NIR SmartProbe Analyzer can be used to determine the purity of methylene chloride (MeCl2 ) solvent recovered for the manufacture of active pharmaceutical ingredients (APIs) (4). Viable cell density or drying processes can also be measured in real time which maximizes time efficiency and reduces material loss due to extensive sample preparation. With little to no sample preparation, NIRS acquires information on both chemical and physical sample properties in each measurement. From the data acquired in a single measurement, multiple parameters can be determined – qualitatively or quantitatively. Because of its short measuring times and the non-destructive nature of its measurements, the full potential of NIRS unfolds, in particular, in process control (5-8).
Atline/Offline Process and Product Development
Metrohm NIRS analyzers allow for monitoring of atline and offline processes like intermediate and product assays or blending and granulation (4, 7, 9). Furthermore, the content uniformity in solid dosage forms (tablets and capsules) can be determined, as well as tablet characteristics like hardness and stability. The NIRS XDS MasterLab has been used to assess content uniformity of chlorpheniramine maleate (CPM), an antihistamine found in common allergy medications. Multiple tablets are loaded and scanned simultaneously with results generated in less than five minutes (9-13).
Quality Assurance of Finished Products
NIRS allows for real-time quality assurance of finished products, such as content determination in creams, gels, tablets, and capsules. In addition, the full transmission spectrometer Metrohm NIRS XDS MasterLab guarantees reliable and highly accurate results when investigating APIs and excipients in tablets (even in blisters) to ensure that chemical and physical properties are verified and purity requirements are met (9-13). Examples include assuring product strength of the anticoagulant heparin sodium, the dissolution profile of the beta-blocker propranolol, and solid dosage form of procainamide HCl used in the treatment of cardiac arrhythmias (10, 11, 14).
Validation of NIRS
Validation processes for use of NIRS ensure compliance with regulatory authorities. Following the published guidelines to develop, validate, submit, and maintain NIRS analytical procedures ensures complete validation and fulfillment of those requirements. While the validation process takes some time and effort, it pays off quickly. Once the software, instrument, and method are validated, NIRS provides rapid, reproducible, real-time monitoring that ensures a high return on investment.
Software Validation
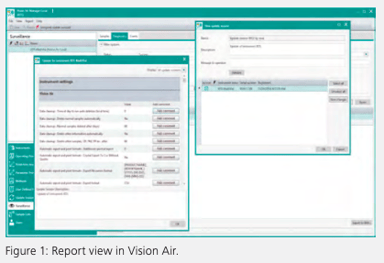 Software that complies with applicable regulations, such as FDA 21 CFR Part 11 (Electronic records; electronic signatures) and/or EU Annex 11 (Computerized Systems), is qualified to be used in any GMP/GLP environment. Metrohm Vis-NIR spectroscopy software Vision Air fulfills all technical requirements mentioned in the FDA 21 CFR Part 11 and the EU Annex (See Figure 1). Vision Air software fulfills requirements for electronic signatures with both customizable and predefined fields and provides for unique log-in and password combinations so that each user maintains appropriate system access. In addition, this software fulfills requirements for electronic record storage and audit trails with its database design, detailed fields for recording instrument and procedural changes, provision of mandatory sample registration fields, and its capability to automatically print, store, and back up records (15).
Software that complies with applicable regulations, such as FDA 21 CFR Part 11 (Electronic records; electronic signatures) and/or EU Annex 11 (Computerized Systems), is qualified to be used in any GMP/GLP environment. Metrohm Vis-NIR spectroscopy software Vision Air fulfills all technical requirements mentioned in the FDA 21 CFR Part 11 and the EU Annex (See Figure 1). Vision Air software fulfills requirements for electronic signatures with both customizable and predefined fields and provides for unique log-in and password combinations so that each user maintains appropriate system access. In addition, this software fulfills requirements for electronic record storage and audit trails with its database design, detailed fields for recording instrument and procedural changes, provision of mandatory sample registration fields, and its capability to automatically print, store, and back up records (15).
Instrument Qualification
The validation process of instruments consists of three phases according to USP<1058> and GMP/GLP: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) as described in Table 1.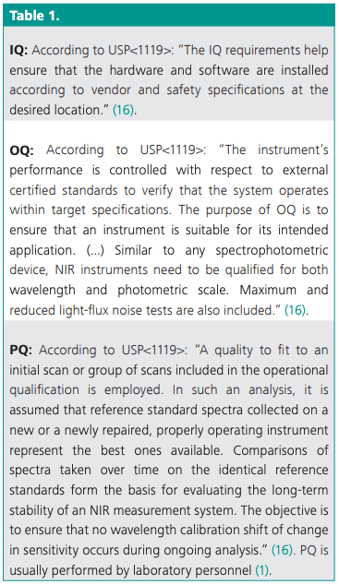
Metrohm Qualification and Validation
Metrohm’s Analytical IQ for NIRS instruments meets all requirements mandated by many governing bodies (USP<1058>, USP<1119>, GAMP, 21 CFR Part 11, PIC/S, etc.). Metrohm offers professional installation and startup of new instruments in compliance with IQ and guarantees that Metrohm NIRS instruments meet OQ requirements, including complete documentation. Metrohm also provides instrument performance certification and subsequent certification for users through work-related training.
Once software and hardware validation are complete, the methods are then developed and validated. According to Ciurczak, “(...) the development laboratory must provide the end user or designated laboratory with the following documentation:
• A written procedure
• A method validation report
• System suitability criteria” (1).
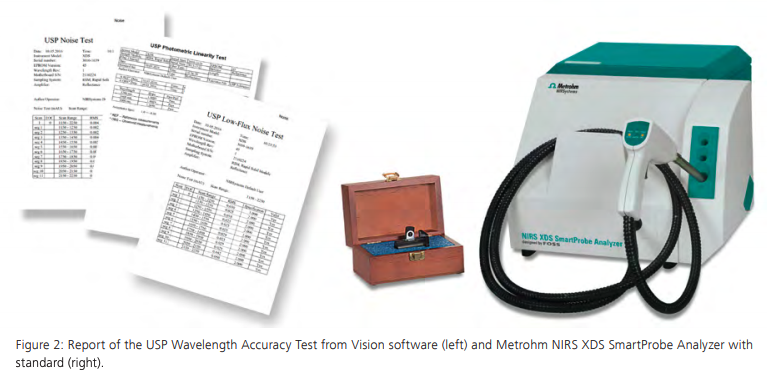
The necessary documentation guarantees the suitability of an analytical procedure when each step of the method development, method validation, and method transfer are clearly documented. Metrohm’s Vis-NIR spectroscopy software Vision Air Complete includes features for method validation. The chemometric software Vision and supported third party tools such as CAMO’s Unscrambler and PLS Toolbox by Eigenvector Research allow for the development and the validation of identification, qualification, and quantification methods (See Figure 2).
Conclusion
Near-infrared spectroscopy (NIRS) is an established secondary analysis method for offline, atline, online, and inline applications in the pharmaceutical industry. Once validated, NIRS offers cost effective and time saving results with little to no sample preparation or destruction. Metrohm NIRS solutions and software enable pharmaceutical analyses that are reliable, compliant with common regulations, and enhanced by support for method and application development.
References
- E.W. Ciurczak and B. Igne, Pharmaceutical and Medical Applications of Near-Infrared Spectroscopy (CRC Press, Boca Raton, Florida, 2015).
- Analysis of pharmaceuticals using near-infrared spectroscopy, Application Bulletin AB-410.
- Identification of 45 aromatic and medicinal plants used in cosmetic and pharmaceutical industry, Application Note AN-NIR-027.
- Monitoring the purity of recovered solvents by NIRS, Application Note AN-NIR-021.
- N. Broad, P. Graham, R. Hailey, A. Hardy, S. Holland, S. Hughes, D. Lee, K. Prebble, N. Salton and P. Warren, Guidelines for the Development and Validation of NearInfrared Spectroscopic Methods in the Pharmaceutical Industry, Handbook of Vibrational Spectroscopy (John Wiley & Sons, Chichester, 2002).
- Robert Mattes et al., Monitoring viable cell density in bioreactors using near-infrared spectroscopy, BioProcessing Journal, 2010.
- Near-infrared spectroscopy for monitoring a single-pot granulator, Application Note AN-NIR-016.
- Analysis of residual moisture in a lyophilized pharmaceutical product by near-infrared spectroscopy (NIRS), Application Bulletin AB-358.
- Following the progress of pharmaceutical mixing studies using near-infrared spectroscopy, Application Note ANNIR- 014.
- Nondestructive, single tablet analysis using the NIRS XDS RapidContent Analyzer, Application Note ANNIR-002.
- NIRS “predictive model” for the release of pharmaceutical active ingredients from solid dosage forms, Application Note AN-NIR-017.
- Near-infrared (NIR) assay and content uniformity of tablets, Application Note AN-NIR-018.
- Determination of active ingredients in solid (pharmaceutical) dosage forms utilizing solid-state standard additions, Application Note AN-NIR-001.
- Quantification of USP heparin units using near-infrared spectroscopy, Application Note AN-NIR-042.
- FDA 21 CFR Part 11 requirements for NIR spectroscopy, White Paper WP-018EN.
- Near-Infrared Spectroscopy, <1119>, USP 39 (2016).
Posted by M. Schilling, N. Rühl